0296
In-vivo validation of T2- and susceptibility-based SvO2 measurements with jugular vein catheterization under hypoxia and hypercapnia1Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States, 3Division of Cardiology, Children’s Hospital Los Angeles, Los angeles, CA, United States
Synopsis
This study aimed to validate T2- and susceptibility-based cerebral venous oxygen saturation (SvO2) measurements with the clinical standard, jugular vein catheterization. T2-relaxation-under-tagging (TRUST) and susceptibility-based oximetry (SBO) were performed on two healthy subjects with jugular catheterization and eleven subjects without catheterization under baseline, hypoxia and hypercapnia. TRUST tightly agreed with the jugular reference under hypercapnia but significantly underestimated SvO2 under baseline and hypoxia. Bias between SBO and the reference was independent on the physiological state. A proportional bias was observed comparing TRUST and SBO. The results suggested caution for inter-subject comparison of absolute SvO2 measurements using either TRUST or SBO.
Introduction
Cerebral venous oxygen saturation (SvO2) is an important parameter for brain oxygenation assessment. Clinical standard for global cerebral SvO2 measurement is using co-oximeter to measure the SvO2 of internal jugular blood sampled through central venous catheters1. Unfortunately, catheterization procedure is highly invasive and unsuitable for broad research use. MRI T2-relaxation-under-tagging (TRUST)2,3 and susceptibility-based oximetry (SBO)4,5 are representative T2- and susceptibility-based methods for noninvasive cerebral SvO2 measurement. Despite increasing applications of these techniques6–11, TRUST and SBO have never been validated against an in-vivo gold standard, nor has their mutual agreement been tested across a broad range of oxygenation levels. Thus, this study investigated the mutual agreement of TRUST and SBO for the quantification of global SvO2 during hypoxia, room air and hypercapnia as well as their agreement with jugular catheterization.Methods
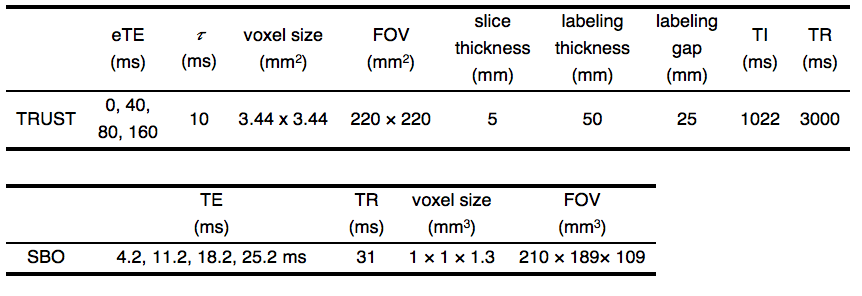
Thirteen healthy subjects (24–35 years) were studied after providing written informed consent under an IRB approved protocol. Blood hematocrit (Hct) was measured on the same day of their MRI scans. MRI was performed on a clinical 3T scanner (Philips Achieva) with a 32-channel head coil. Subjects were placed on a 2-liter reservoir rebreathing circuit and imaged under three oxygenation conditions: 1) hypoxia (12% O2 and 88% N2), 2) hypercapnia (5% CO2 and room air), and 3) baseline (room air). Eleven subjects were scanned without catheterization under these three oxygenation conditions. Test-retest agreement was assessed under baseline.
Two subjects were scanned after internal jugular vein catheterization with 3-French tracker catheter. During the entire imaging session, blood was drawn from the catheter every 3 min by a cardiologist for SvO2 measurement using a portable co-oximeter (Accriva Diagnostics, Avoximeter 4000). The co-oximeter measurement was used as ground truth. The sequence (Table 1), calibration model and image processing used for TRUST were provided by Lu et al2. Following a recent system upgrade, the post-saturation module in the TRUST sequence12 was inadvertently deactivated, forcing us to retrospectively correct the resultant T2 underestimation via Bloch simulation. SBO data were acquired using a 3D multi-echo GRE sequence (Table 1) with full flow compensation13 and processed in a 3D manner (Figure 1). The magnetic field shift inside the vessel,, was converted to SvO2 using:
where ppm, and is the vessel tilt angle with respect to the main B0 field.
Results
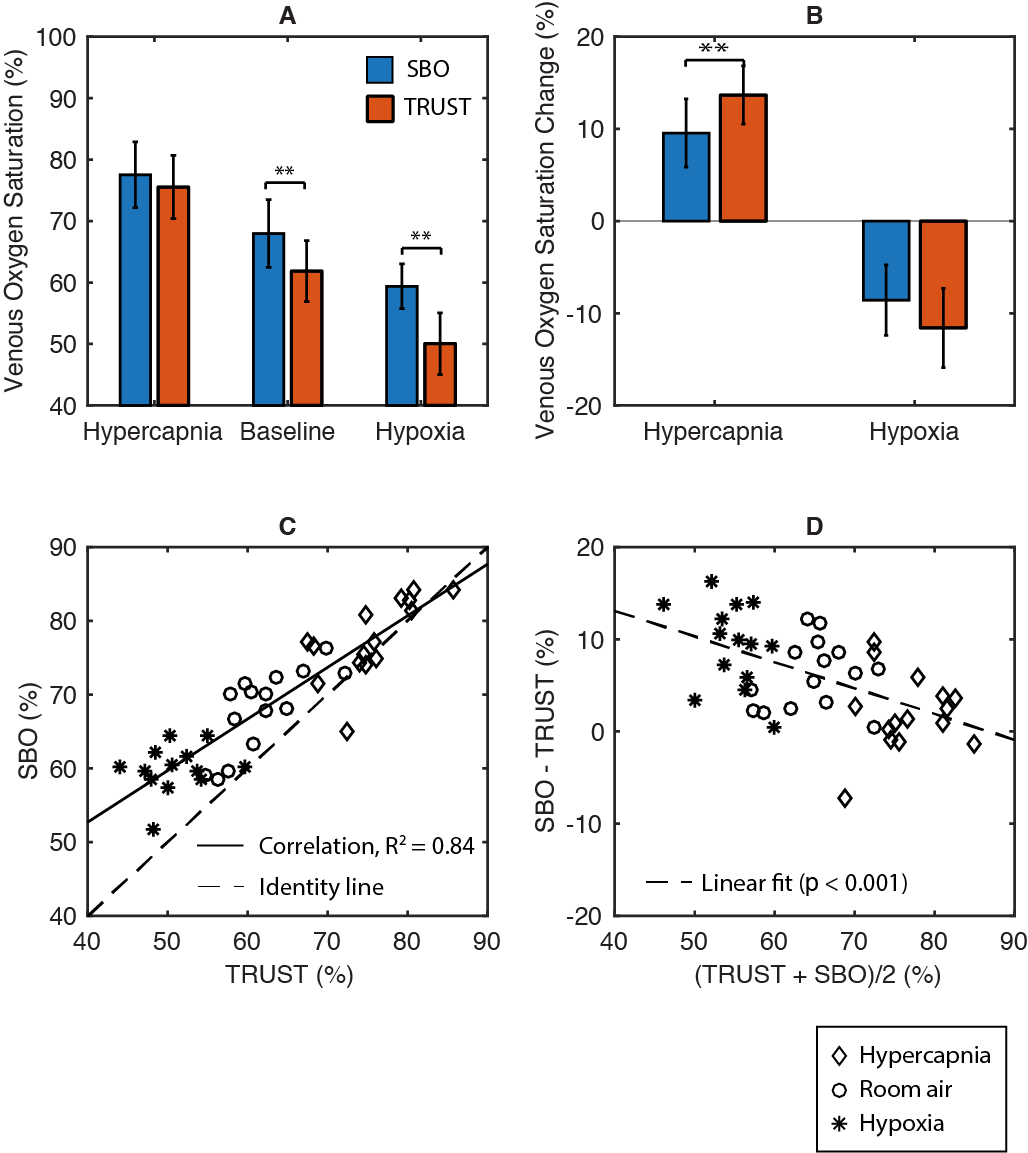
TRUST and SBO had excellent test-retest agreement of 1.4% and 2.2%. In Figure 2, SvO2-TRUST and SvO2-SBO were highly correlated (Pearson r = 0.91, R2 = 0.84). However, Bland-Altman analysis revealed a significant proportional bias (p < 0.01). Compared with SBO, TRUST presented greater response of SvO2 to stimulus, particularly for the hypercapnia challenge.
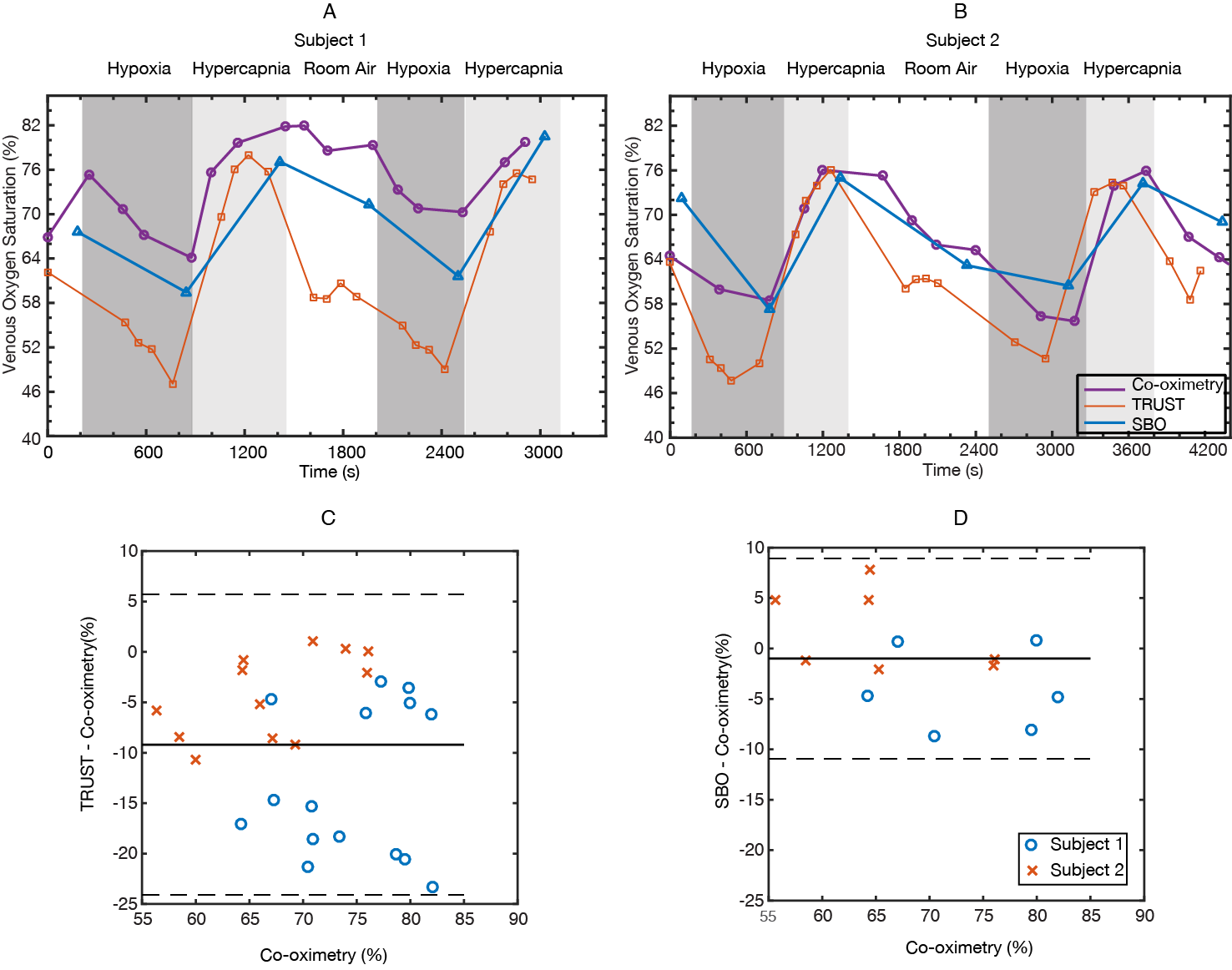
In both subjects scanned with jugular catheterization, SvO2-TRUST measurements closely matched the jugular reference under hypercapnia but were lower than reference under hypoxia and baseline (Figure 3A-B). In contrast, the discrepancy between SvO2-SBO and reference was independent on the physiological states. On average, the bias between SvO2-TRUST and the reference was -9.2% (p < 0.0001), while the bias between SvO2-SBO and reference was -1.0% (p = 0.45) (Figure 3C-D).
Discussion
The proportional bias between TRUST and SBO has been suggested by several prior studies10,14, but we demonstrated it more convincingly with the broad range of oxygenation produced. The large discrepancy between TRUST and the jugular reference is unlikely to relate to physiological distinction between the two veins. The saturation difference between jugular vein and SSS has been well characterized (only 2-3% at baseline15). We suspect that the TRUST bias may originate from the calibration models themselves3,16,17, which were obtained from in-vitro blood experiments that may fail to mimic the changing venous blood environment under gas challenges (e.g. venous pCO2 varied from 30-55 torr in this experiment).
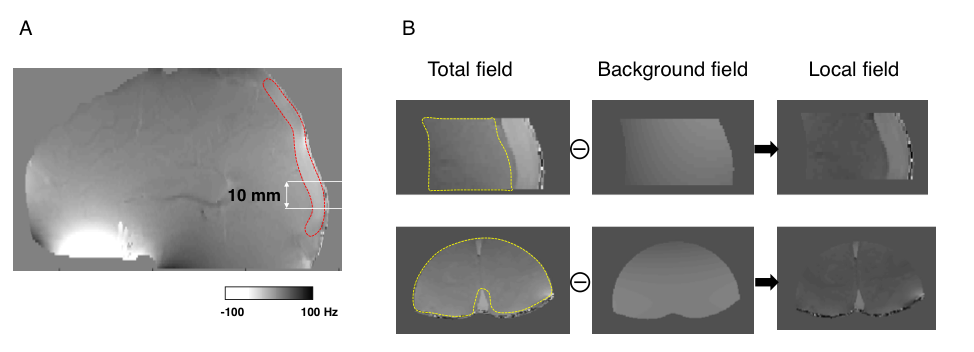
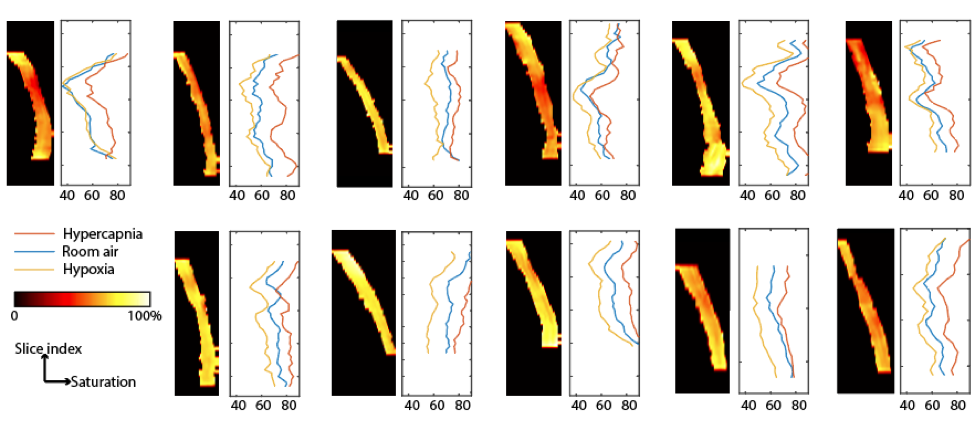
Although SBO had smaller bias from the jugular reference compared to TRUST, SBO has major challenges for use in SSS. The acquisition of 3D multi-echo phase data allowed us to perform 2D SBO processing for all axial slices with vessel tilt < 20°. The predicted SvO2 values varied by an absolute saturation range of 22% along the slice direction (Figure 4). Such high variance along the slice direction will lessen the confidence of using 2D SBO processing for absolute SvO2 quantification.
Conclusion
In-vivo validation with jugular catheterization suggested that TRUST underestimated SvO2 under room air and hypoxic conditions. A systematic bias was observed between T2- and susceptibility-based methods that depends on the oxygenation state. The results suggested that caution should be taken for inter-subject comparison of absolute SvO2 measurements using either TRUST or SBO.Acknowledgements
This work is supported by the National Heart Lung and Blood Institute (1RO1HL136484-A1, 1U01HL117718-01), the National Institute of Clinical Research Resources (UL1 TR001855-02) and by research support in kind from Philips Healthcare.References
1. Schell, R. M. & Cole, D. J. Cerebral Monitoring: Jugular Venous Oximetry. Anesth. Analg. 90, 559–566 (2000).
2. Lu, H. & Ge, Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn. Reson. Med. 60, 357–363 (2008).
3. Lu, H. et al. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn. Reson. Med. 67, 42–49 (2012).
4. Langham, M. C., Magland, J. F., Epstein, C. L., Floyd, T. F. & Wehrli, F. W. Accuracy and precision of MR blood oximetry based on the long paramagnetic cylinder approximation of large vessels. Magn. Reson. Med. 62, 333–40 (2009).
5. Jain, V., Langham, M. C. & Wehrli, F. W. MRI Estimation of Global Brain Oxygen Consumption Rate. J. Cereb. Blood Flow Metab. 30, 1598–1607 (2010).
6. Xu, F., Liu, P., Pascual, J. M., Xiao, G. & Lu, H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J. Cereb. Blood Flow Metab. 32, 1909–18 (2012).
7. Xu, F. et al. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J. Cereb. Blood Flow Metab. 31, 58–67 (2011).
8. Bush, A. M., Coates, T. D. & Wood, J. C. Diminished cerebral oxygen extraction and metabolic rate in sickle cell disease using T2 relaxation under spin tagging MRI. Magn. Reson. Med. (2017). doi:10.1002/mrm.27015
9. Ge, Y. et al. Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI. J. Cereb. Blood Flow Metab. 32, 403–12 (2012).
10. Rodgers, Z. B., Englund, E. K., Langham, M. C., Magland, J. F. & Wehrli, F. W. Rapid T2- and susceptometry-based CMRO2 quantification with interleaved TRUST (iTRUST). Neuroimage 106, 441–50 (2015).
11. Rodgers, Z. B., Jain, V., Englund, E. K., Langham, M. C. & Wehrli, F. W. High temporal resolution MRI quantification of global cerebral metabolic rate of oxygen consumption in response to apneic challenge. J. Cereb. Blood Flow Metab. 33, 1514–22 (2013).
12. Xu, F., Uh, J., Liu, P. & Lu, H. On improving the speed and reliability of T2-relaxation-under-spin-tagging (TRUST) MRI. Magn. Reson. Med. 68, 198–204 (2012).
13. Xu, B., Liu, T., Spincemaille, P., Prince, M. & Wang, Y. Flow compensated quantitative susceptibility mapping for venous oxygenation imaging. Magn. Reson. Med. 72, 438–445 (2014).
14. Barhoum, S. et al. Method for rapid MRI quantification of global cerebral metabolic rate of oxygen. J. Cereb. Blood Flow Metab. 35, 1616–22 (2015).
15. De Vis, J. B., Lu, H., Ravi, H., Hendrikse, J. & Liu, P. Spatial distribution of flow and oxygenation in the cerebral venous drainage system. J. Magn. Reson. Imaging 47, 1091–1098 (2018).
16. Bush, A. et al. Empirical model of human blood transverse relaxation at 3 T improves MRI T2 oximetry. Magn. Reson. Med. 77, 2364–2371 (2017).
17. Liu, P. et al. T1 and T2 values of human neonatal blood at 3 Tesla: Dependence on hematocrit, oxygenation, and temperature. Magn. Reson. Med. 75, 1730–1735 (2016).
Figures